Anovo Early Access Program
Now the exclusive benefits of Anovo (AnovoRx) rare and orphan drug support can begin with your EAP.
A continuous pathway to program success
![]()
Eliminate the inefficiencies of separate providers for your Early Access Program and your commercial distribution strategy.
![]()
When you choose Anovo as your exclusive provider for both, we use the EAP to develop a working model of your commercial program. That means you’ll be ready to hit the ground running the moment your therapy is approved.
![]()
No handoffs. No data transfers. No loss of expertise in your product. No disruption for your patients. No reinventing the wheel.
And as both your early access and commercial partner, Anovo is as invested in your product’s long-term success as you are – starting with your first patient.

“One of the aspects we noticed right away [after switching to Anovo] was how seamlessly the process flowed. When setting up an EAP with large institutions, there are always complexities to navigate, but Anovo’s approach made it easy to address needs and respond quickly.”- Egetis Therapeutics
The unique power of a single provider
Erase the space between early access and commercial distribution. Anovo’s dedication to orphan drug support carries your patients and products seamlessly from the investigational phase to the commercial phase.
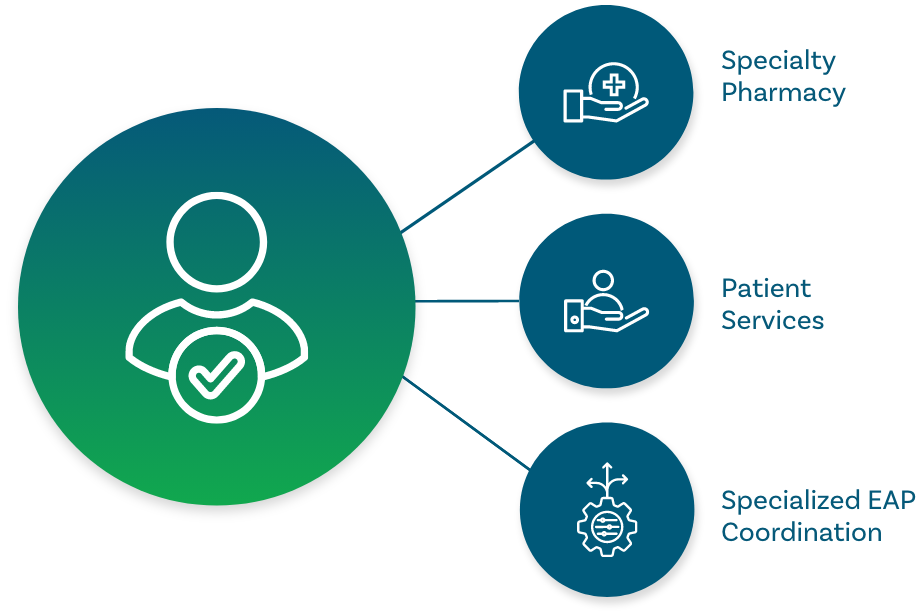
Give your early access patients the highest standard of care
Anovo provides the same market-leading, holistic rare disease support we provide for approved therapies.
Advance toward commercialization at every step
With our scalable resources and responsive culture, we steadily evolve your Early Access Program to prepare for the day your therapy receives approval.
Throughout your product’s journey, Anovo captures insights, optimizes workflows, resolves issues, discovers actionable opportunities, and customizes standard operating procedures (SOPs) in response to the latest patient insights.
Transition seamlessly
By the time the FDA approves your therapy, your commercial implementation program is already in place with Anovo.
You keep moving forward on a continuous path, without the risks of losing something in translation with a new provider. The specialty pharmacy and patient services teams who became product experts during the EAP, keep working with your patients – and your patients continue to benefit from our exclusive, all-in-one model.
News: A Partnership to Enhance Neurodegenerative Disorder Care
In 2024, Anovo and Synapticure established a partnership designed to improve support for patients with rare neurodegenerative disease diagnoses.
Our two companies share deep expertise in these conditions and a commitment to equitable access to the drugs that treat them.
Synapticure manages and facilitates expanded access programs for rare and orphan neurological therapies, often in collaboration with traditional Clinical Research Organizations (CRO).
One of Synapticure’s outstanding capabilities is the expert delivery of complex therapies wherever a patient lives, including areas where access to clinical expertise and these life-saving treatments is limited.
By combining our strengths, we ensure access while providing patients and manufacturers with Anovo’s comprehensive EAP support system. This enables optimal patient care and a smooth transition to commercialization.
It starts with a conversation. Contact us today.
